Zhang Jiagang Medical Device High-tech Industrial Park
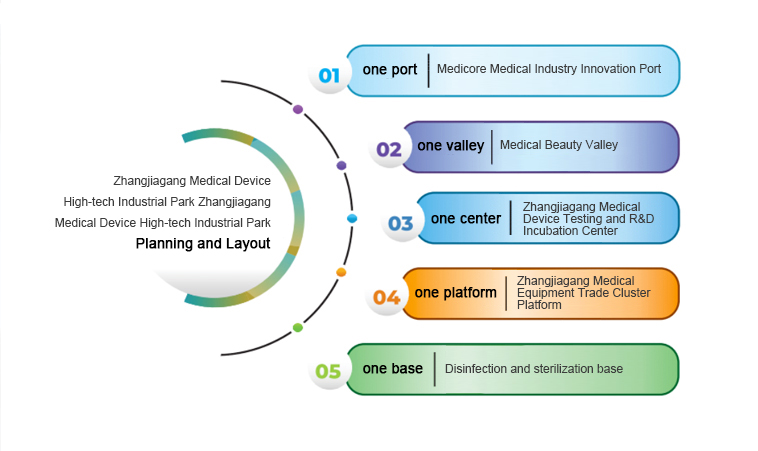
Zhangjiagang Medical Device High-tech Industrial Park was established in February 2019 with the approval of Zhangjiagang Municipal People's Government and was listed as one of the first batch of biomedical industrial parks in Suzhou by Suzhou Municipal People's Government in the same year. The total planned area of the park is 5,785 mu, with a core area of 1,880 mu. The first phase of the industrial park has been developed and constructed with a total investment of 5 billion yuan. The second phase of the industrial park is planned to be constructed with 1,200 mu, with a total planned investment of over 10 billion yuan. Zhangjiagang Medical Device High-tech Industrial Park focuses on the goal of building a "thousand, hundred, and ten" medical device industrial base, and deeply deploys a new model of full industrial chain development of "one port, one valley, one center, one platform, and one base", namely, the Innovation Port, Medical Beauty Valley, Testing Center, Medical Trade Platform, and Sterilization Base, to strengthen the high-end medical industry chain and build a high-end medical device industry base integrating R&D, incubation, testing, registration, production, sterilization, and trade, to create the most leading and recognizable "most complete industrial chain" and "strongest industrial cluster" in China, to achieve more than 1,000 companies settled in, achieve invoice sales of more than 10 billion yuan, and collect more than 1.5 billion yuan in taxes.

Medicore Industrial innovation Port
Located in Zhangjiagang Metallurgical Park (Jinfeng Town), Suzhou City, Jiangsu Province, it is a major project at the municipal level in Suzhou, with a total investment of more than 3.8 billion yuan, covering an area of 136.5 mu and a construction area of about 170,000 square meters. Relying on the development layout of Zhangjiagang Medical Device High-tech Industrial Park, the Medicore Medical Device Industry Innovation Port has built a medical device closed-loop ecological industry chain integrating medical device innovation and R&D center, achievement transformation center, testing center, public experimental platform, CDMO platform, and investment and financing platform. This development model of one body, two wings and three supporting facilities provides strong industrial support and development momentum for Medicore.

Medical device testing and R&D incubation center
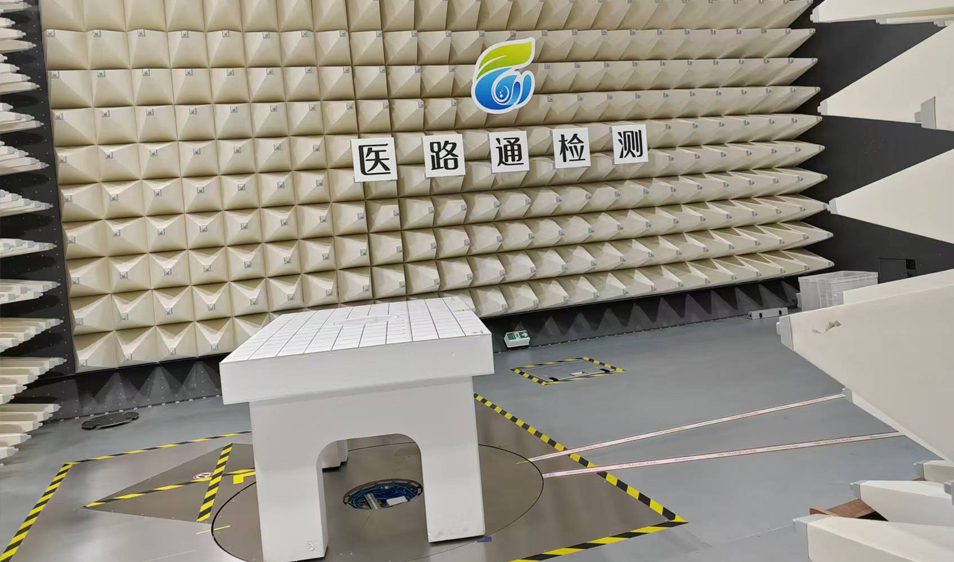
Zhangjiagang Medical Device Testing and R&D Incubation Center (abbreviated as Yilutong Testing) is located in Zhangjiagang Medical Device High-tech Industrial Park and serves as an important industrial supporting technology platform in the park. In accordance with the overall idea of "serving enterprises, assisting investment promotion, and growing together", Yilutong Testing provides enterprises with a series of testing services such as registration testing, commissioned testing, pre-testing and R&D testing by utilizing the expert resources of testing institutions and scientific research institutes. The laboratories that have been built and put into use include electrical safety laboratories, electromagnetic compatibility laboratories (two semi-anechoic chambers of 10-meter method and 3-meter method), physical and chemical analysis laboratories, microbiological laboratories, medical robot performance laboratories, optical performance laboratories, usability evaluation laboratories, environmental reliability testing laboratories, etc. With the dual qualifications of national CMA qualification certification and CNAS laboratory accreditation, Yilutong Testing can issue test reports with market credibility for medical device companies that meet the requirements of medical device registration regulations.
- 01
Zhang Jiagang Medical Device High-tech Industrial Park
- 02
Medicore Industrial innovation Port
- 03
Medical device testing and R&D incubation center