Our company
About us
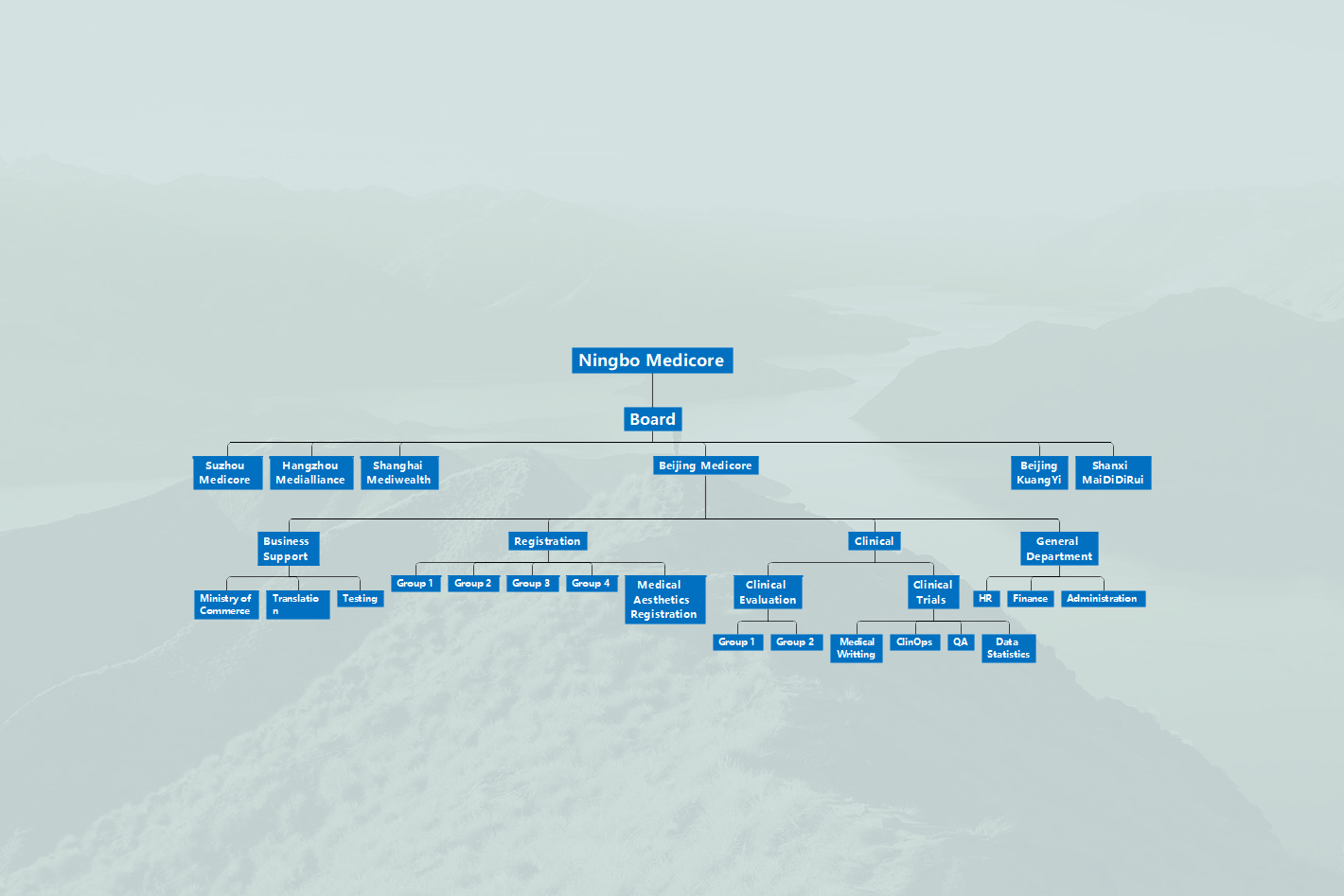
A first-class domestic medical service company, wholly owned by Ningbo Medicore Pharmaceutical Technology Co., Ltd. We have a deep professional background and we provide medical product technical consulting services based on medical product registration. We bring together outstanding talents and multiple resources, work closely with many medical institutions, and committed to providing high-quality and professional services to many medical companies around the world.
Contact number: 010-65516258
Medicore
Text
Text
">Medical regulatory consulting service provider, committed to providing high-quality regulatory consulting services to global customers.

Industry experience
Text
1" data-key="items">Focusing on medical device registration and clinical CRO for 20 years, has helped thousands of domestic and foreign medical device manufacturers to obtain medical device registration certificate.

Service team
Text
1" data-key="items">Has a professional service team of 200 people, the team has rich experience in medical device registration and clinical trials, clinical evaluation, and medical device market access.

Concentration field
Text
1" data-key="items">Mainly focusing on high-risk passive implant interventional products such as aesthetic medicine, dentistry, orthopedics, and cardiovascular; high-end manufacturing and IVD fields such as high-frequency active equipment, artificial intelligence, and navigation robots.

Service Distribution
Text
1" data-key="items">Headquartered in Beijing Chaoyang Yuanyang Guanghua International, it has wholly-owned subsidiaries in Shanxi, Shanghai, Hangzhou, Suzhou and other places.
Core Value
Comprehensive services, One-stop, Professional, Effective